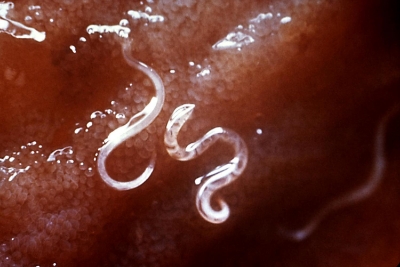
We’ve recently talked about a few articles that have studied helminth infection with respect to the microbiome, and how these infections could possibly confer some therapeutic benefits. Another recent study conducted by researchers at Duke University reinforces these findings. Autoimmune and inflammatory disorders appear to be more common in developed societies, and many have suggested that the microbiome is a major driver of these changes to our immunity. These investigators wanted to assess whether or not helminths – which have a lot of influence on the immune system – had any effect in modulating the brain immune system in the context of living conditions and early-life infection, as this has been shown to result in neurodevelopment disorders.
In this study, male and female rats were infected with a H. diminuta cystercircoid rat tapeworm a few weeks prior to breeding. The rats were segregated by living conditions, housed in either dirty colonies (or “farm-like” environments), where no water or air filtration was provided) or standard clean pathogen-free laboratory conditions. The offspring in both environments were delivered helminths, and the males were infected with E. coli early in life.
Later in adulthood, the immune systems of the progeny animals were challenged by lipopolysaccharide (LPS) inductions in learning tests, and brains were collected shortly after to examine changes in molecular immune responses. Exaggerated immune responses were observed in rats that were infected with E.coli early in life in the standard clean lab conditions. Alternatively, the cohort that lived in the farm-like conditions did not experience an increase. Both groups were infected with helminths.
To narrow down further, the researchers examined the impact of helminths alone in rats housed under clean pathogen-free laboratory conditions. Indeed, cytokine responses in rats infected with E.coli were reduced in the animals whose mothers were infected with helminths before giving birth. In addition to immunologic modulation, helminth infections in adult rats where shown to reduce memory deficits that are common following E. coli infection, suggesting helminth infection played a role in modulating developmental disorders due to bacterial infection.
The helminths also had an effect on the microbiomes of the rodents. 16s rRNA sequencing revealed an average 25% shift in microbiome composition of animals infected with helminths (with a predominant shift of Bacilli to Clostridia). Rats that were infected with E. coli early in life experienced a microbiome composition shift in adulthood, as more harmful Bacteroidetes species were found in adults. Interestingly, this observation was not found in those who were E.coli infected but also infected with helminths, suggesting helminths prevented this composition shift.
Overall, these findings suggest that helminths could provide therapeutic benefit, especially after infection early in life. It will be interesting to see how this research can translate to human models, especially by narrowing down bacterial infections that could harm or benefit development. Understanding what drives these developmental complications could have immense health benefits for the public.