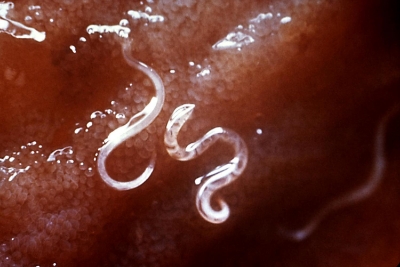
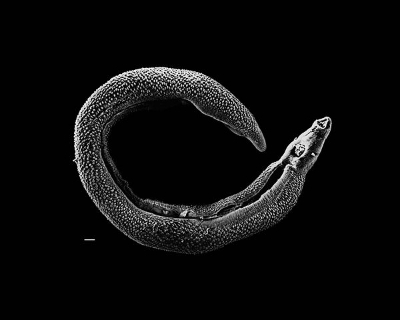
We’ve talked about how helminths can impact health, highlighting many studies that describe relationships between helminth infections, disease, and industrialization versus underdevelopment. Asthma is a condition prevalent particularly in industrialized/westernized societies, and helminths have been shown to directly regulate immune responses and are linked with a reduction in asthma prevalence. Correspondingly, there is less incidence of asthma in underdeveloped societies. Additionally, helminth infections are typically associated with compositional shifts in intestinal microbiota. A large research team sought to investigate the potential role of intestinal microbiota in modulating helminth-induced allergic inflammation, postulating that there is indeed significant cross-talk between the microbiome and helminths as opposed to intrinsic inflammatory responses to helminths.
The researchers first determined that helminth infection can reduce the severity of allergic airway inflammation in mice. Mice were infected with Heligmosomoides polygyrus bakeri (Hpb) murine-specific helminths and then exposed to house dust mite to induce allergic inflammation. The mice exposed to the Hpb helminth demonstrated a reduction in severity of inflammation. Next, the researchers were able to demonstrate that intestinal bacteria play a role in helminth’s modulatory role of allergic airway inflammation. Antibiotics were administered to Hpb-infected mice to eliminate intestinal microbiota populations. Helminths infection reduced inflammation, but did not attenuate inflammation in anti-biotic treated mice, even though total worm-count was similar between both groups (antibiotic group and non-antibiotic group).
Helminth exposure and subsequent shifts in microbiota composition and biochemical activity was then examined. 16S sequencing revealed that Hpb-infected mice induced an outgrowth of Clostridiales bacteria. Increases in small-chain fatty-acid (SCFAs) were also observed in Hpb-infected mice, a likely outcome of a shift in bacterial community structure. Interestingly, the microbiota of a Hpb-infected mice were transferred to naïve mice, and this was sufficient in protecting against allergic inflammation, further confirming the microbiome’s modulatory roles. Previous reports have pointed to regulatory T cells (Tregs) as responsible for regulating immune/inflammatory responses, and in this study the researchers demonstrated Treg involvement. Furthermore, helminth-induced Treg suppression and anti-inflammatory activity was observed, mediated by a G-protein receptor entitled GPR-41.
Together these findings further elucidate the role of helminths in disease, while uniquely pointing to the gut microbiome as a critical mediator of this interaction. Learning more about these relationships can help us better understand broad epidemiology trends associated with helminth infection and human health.