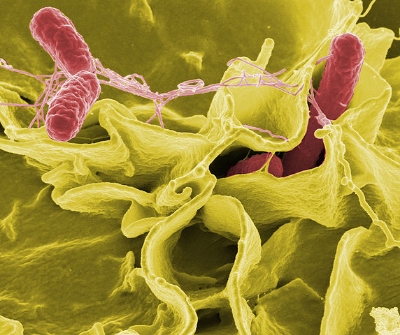
Microbiota that populate the gut do not have access to lymphatics, the liver, or other peripheral tissues in healthy individuals. The gut-vascular barrier (GVB) plays an essential role in limiting bacteria access to systemic circulation in addition to protecting against pathogenic invasion. However, some pathogenic bacteria can reach these tissues and induce a systemic immune response, but how this is exactly occurs is unknown. Researchers from Italy sought to address this by exploring how GVB disruption lends itself to bacterial-pathogenesis in liver and lymphatic tissue sites.
Two groups of mice were analyzed, one healthy control group and one group infected with Salmonella, a bacteria selected to study the pathogenic effects on the GVB. Healthy control mice were first analyzed using fluorescent dextran polymer dyes at different sizes to assess the leakage of the dye into the intestines for visualization and characterization of the GVB. After injection, intestinal blood vessels were analyzed using two-photon microscopy, and it was observed that an endothelial barrier could discriminate particles that are functionally similar according to size. Specifically, dextran polymers 4 kilodaltons (kD) in size could enter the blood stream freely, while dextran polymers 70 kD in size could not. The same experiment was then repeated in mice who were administered Salmonella prior to dextran, and interestingly the 70 kD polymers diffused freely into the blood stream, suggesting that the Salmonella disrupted the GVB. Exploring deeper, the experimenters characterized the endothelial cell types that formed this tight-junction barrier, as it was found that plasma-lemma vesicle-associated protein-1 (PV1) was up-regulated in blood 6 hours after being exposed to Salmonella. Furthermore, this finding was associated with systemic damage present in other organs.
In another experiment using the dextran dye, the researchers determined that the dye reached the liver in mice infected with Salmonella after 60 minutes following injection, as it was concluded that the Salmonella traveled to the liver via portal vein circulation as opposed to lymphatic transport (where it would be present in the spleen). Lastly, it was determined that Salmonella could discretely regulate B-catenin signaling by blocking these signals from being released in vascular endothelial cells.
These findings highlight important mechanistic features of a physiological construct potentially implicated in many pathogenic diseases. In the context of liver damage and celiac disease, these findings could link the GVB as drivers of pathogenesis as the GVB is disrupted in celiac patients. Disruptions to the GVB deserve more investigation as this physiological barrier could be implicated in many pathogenic diseases.